Here are some tips for cell culture that will hopefully help you keep a well organized lab and contamination free environment for successful experiments:
Clean tissue culture hoodTo clean air and beyond
Turn on the hood for at least 15 minutes before you start any tissue culture work and make sure that clean air is flowing in. The vent for the in-flowing air is normally visible and you should always ensure nothing is covering it. Turning on the UV light 3-4 times a week for up to half an hour can help keep your hood contamination free, but you should keep in mind that UV light only kills microorganisms that it strikes directly. UV light exposure is also harmful for eyes and skin so keep the light off when the hood is in use.
Keep your contaminants away
While it’s likely that you’re the main source of contamination for your experiments, the bright side is that you also have the power to take the added precautions necessary to keep your cells from becoming contaminated. Wipe down everything that’ll be brought under the hood with 70% ethanol, including your glove-covered hands! You should additionally remove all jewellery as well as that watch you’re wearing before you start. Also, avoid unnecessary talking because talking generates microbe-laden aerosols that may enter the hood. Planning your experiments thoroughly before entering the hood is another great way to prevent contamination. This sounds rather trivial, but often solves most problems before they occur. With proper planning, you’ll know exactly what materials you’ll need to bring into the hood and can wipe them all down before you start. This will also help you avoid moving things in and out of the hood while work is in progress.
Declutter! Keep the hood clean
Hoods should not be used as storage cabinets. Clutter not only makes it difficult to clean your workspace properly, but can also disrupt the laminar air flow around your work area. Make sure to only bring required materials into the hood. This will give you enough space to move around freely without causing spills. Also, keep all liquid containers closed when they aren’t in immediate use. This will prevent spills. Finally, avoid sticking pipettes into solution bottles directly, rather decant the solutions into disposable sterile tubes first. Not only does this make things more manageable, but it also keeps stock solutions from getting contaminated.
Make sure cells don't stay out too long
While you’re preparing things, make sure your cells don’t stay out too long. Cells usually have their comfort zone around 37 °C and 5% CO2 and get agitated with sudden changes in temperature (especially if you have air conditioning on) so leave them in a sterile incubator. This also means that any medium or washing buffer, such as PBS (Phosphate Buffered Saline), that comes in contact with the cells should already be at 37 °C. Most labs have a water bath constantly at 37°C for this purpose.
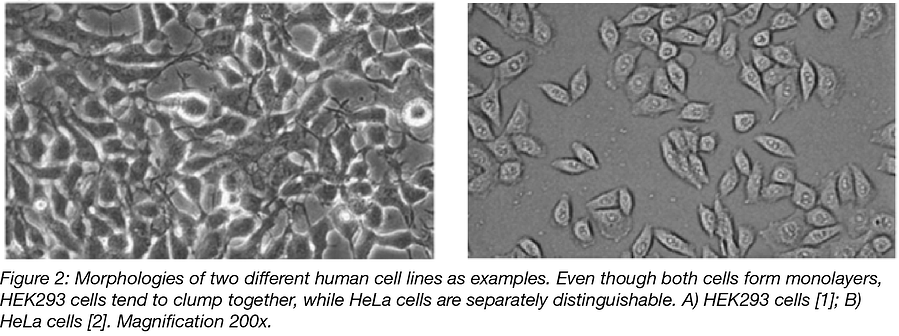
Cell morphology: Shape and size matter
You should have a light microscope close to the hood so you can view your cells regularly (both before entering the hood and when putting them into the incubator). It also helps to get familiar with the morphology of the cell line you’re working with so that cross-contamination can be identified. Even if some mammalian cells lines have similar morphologies, being familiar with these morphologies and doing a quick check under the light microscope will help you identify bacterial and yeast contaminants.
Set up a routine to split cells
Don’t be lazy about splitting cells, instead try to form a routine. Twice a week often works for most fast-growing cell lines (such as HEK293, which multiplies every 16 hours). So if you, for example, split cells on a Monday diluting them 1:10, you should be able to split on the following Thursday, or, at the latest on Friday. It’s best to split cells before the plates are more than 80-85% confluent because cells like their space. If they reach confluence, they stop growing (contact inhibition) and it takes them time to recover after they are passaged. This could change their growth rates and morphology, rendering their continued use inadvisable for experiments requiring reproducible results. Most cell culture media contain the pH indicator phenol red (pH range 6.0 (yellow) - 8.0 (red)). Blood pH normally lies around 7.35-7.4 so mammalian cells are sensitive to pH. Anything between orange and light orange usually indicates that the cells have grown enough, are making their surrounding media acidic through the secretion of lactic acid (a by-product of cellular metabolism that is toxic to the cells), and need to be split very soon.
There is power in numbers!
When starting off with a new cell line, grow the cells, aliquot them into a number of vials, freeze them, and store all but one in liquid nitrogen. The frozen vials make up your master stock, while you can use the remaining vial to grow and freeze additional cells that can be used as your working stock. This way you can go through your working stock vial by vial before thawing another vial of the master stock. This ensures that you have many cells stored in their original condition and can replace your working stock if it gets too old or contaminated. This also ensures that anyone needing new cells in the lab can take a vial of the ‘mint condition’ master stock instead of ‘borrowing’ cells that have already been passaged many times and could be contaminated.
Keep track of passage numbers
Keep an eye on the passage number. Cells change their growth rate and morphology at high passage numbers (>30-40 times), depending on the cell line so old cells should be retired! Conversely, cells younger than the 3rd – 4th passage are too young and should not be used for important transfection experiments right after they’ve been thawed. Additional passages will allow your cells to achieve a consistent growth rate.
different shades of phenol red pH indicator in DMEM cell culture medium
For every minute spent organizing, an hour is earned (Benjamin Franklin)!
It’s good practice to label your plates and/or flasks with your name, date, the cell line you’re using and its passage number. Especially in a lab where multiple people are working on different things, this will help you avoid cross-contamination through accidental mixing and will also help you keep good records of your experiments.
Autoclave is the rave!
Last, but not least, any lab material that is intended for reuse (e.g. Pasteur pipettes for liquid waste) should be sterilized before each use. Also, all contaminated liquid and solid waste should be autoclaved before being disposed
Happy culturing!
Medicalet can provide you with the best Cell and Tissue culture product. Medicalet laboratory equipment is outstanding in terms of innovation, ergonomic, design and functionality, providing worldwide users efficiency and comfort in their daily work.
If you are interested in our products, please feel free to contact us and ask for samples!
